Revised: October 8, 2013
Accepted: October 17, 2013
Published online: December 28, 2013
Processing time: 201 Days and 11.1 Hours
In spite of a documented reduction in incidence in high-income countries over the last decades, stroke is still a leading cause of death and disability worldwide. With the ageing of the population stroke-related economic burden is expected to increase, because of residual disability and its complications, such as cognitive impairment, high risk of falls and fractures, depression and epilepsy. Furthermore, because of the substantial rate of early and long-term vascular recurrences after the first event, secondary prevention after cerebral ischaemia is a crucial issue. This is even more important after minor stroke and transient ischaemic attack (TIA), in order to reduce the risk of potentially more severe and disabling events. To accomplish this aim, acute long-term medical and surgical treatments as well as lifestyle modifications are strongly recommended. However, apart from the well-established indications to thrombolysis, studies in acute phase after a first stroke or TIA are scarce and evidence is lacking. More trials are available for long-term secondary prevention with different classes of drugs, including antithrombotic medications for ischaemic events of arterial and cardiac origin, especially related to atrial fibrillation (antiplatelets and anticoagulants, respectively), lipid lowering agents (mainly statins), blood pressure lowering drugs, surgical and endovascular revascularization procedures.
Core tip: Aggressive and combination treatments in the acute phase after transient ischaemic attack or minor stroke have been shown to be beneficial in few studies, but results of ongoing randomized trials are required. On the other side, in long-term prevention the most important innovation is the advent of new anticoagulant agents for stroke prevention in atrial fibrillation. Recent trials showed efficacy and safety of thrombin and factor Xa inhibitors, compared to vitamin K antagonists, whose use is hampered by several limitations. These new drugs will potentially increase the number of patients treated according to guidelines, thus preventing a remarkable proportion of strokes.
- Citation: Volonghi I, Padovani A, Zotto ED, Giossi A, Costa P, Morotti A, Poli L, Pezzini A. Secondary prevention of ischaemic stroke. World J Neurol 2013; 3(4): 97-114
- URL: https://www.wjgnet.com/2218-6212/full/v3/i4/97.htm
- DOI: https://dx.doi.org/10.5316/wjn.v3.i4.97
Stroke is a leading cause of death and disability worldwide[1] and its burden on global health-care expenditure is supposed to increase, mainly because of the ageing population. Incidence of stroke is higher than that of myocardial infarction in some regions[2] and in spite of a mild reduction of incidence in developed countries in the last four decades, a rise of cerebrovascular events has been observed in low-income countries[3]. Stroke has also substantial indirect costs related to complications, such as post-stroke dementia, depression, falls, fractures and epilepsy.
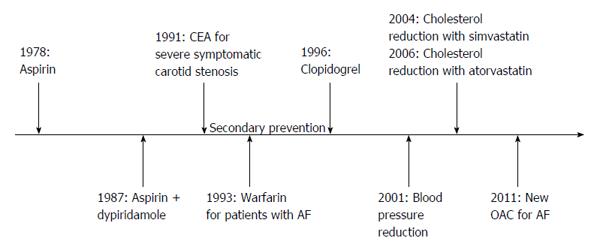
Almost 90% of all cerebrovascular events depends on few medical conditions, including bad habits (e.g., smoking, physical inactivity, diet, alcohol excess, stress) as well as arterial hypertension, cardiac diseases, diabetes, and high ratio of apolipoprotein B to apolipoprotein A1[4]. Lifestyle modifications as well as medical and surgical preventive strategies may, therefore, reduce the risk of stroke. Although primary prevention remains a milestone, effective secondary prevention plays also a fundamental role in this regard. About 30% of strokes occur in individuals with a previous transient ischaemic attack (TIA) or stroke[2], and more than 50% occur in subjects with previous vascular events of any kind[5]. Much progress has been made in the last decades in stroke prevention, and there is now evidence from randomized trials that several treatments are effective in preventing recurrences (Figure 1). In particular, at least 80% of recurrent events might be prevented with the use of a comprehensive approach that includes dietary modifications, physical exercise, blood-pressure lowering drugs, antiplatelet therapy, and statins[6].
The effect of all these different interventions on stroke risk can only be derived from population-based studies. In this regard, it has been shown that the risk of recurrent stroke in all patients in a United Kingdom population-based study after a first-ever TIA or non-disabling stroke in the period 1981-1986 was higher than the risk of the same population during 2002-2010[5]. These findings were corroborated by a recent review[7] of randomized controlled trials in secondary prevention after stroke and vascular events. Recurrent stroke and vascular events rates declined substantially in the last 5 decades, mostly because of improved blood pressure (BP) control and more frequent use of antiplatelet therapy.
In this review we will focus on medical treatment for secondary prevention in patients with first-ever TIA or ischaemic stroke and separate acute secondary prevention (i.e., prevention in the first hours or days) from long-term secondary prevention. We will also deal with surgical procedures and endovascular techniques for prevention of recurrences in carotid stenosis as well as therapeutic strategies in stroke associated with patent foramen ovale (PFO).
The risk of recurrent stroke is higher during the first 90 d after an index event, longitudinal studies showing that approximately 1 out of 2 recurrences in the first year post-stroke occurs within the first 90 d[8-12]. The 90-d risk of recurrence after a TIA has been reported to be as high as 17%, with the greatest risk in the first week[13,14]. In this regard, prospective studies have shown that stroke risk after a TIA or minor stroke is 3.1% (95%CI: 2.0-4.1) at 2 d and 5.2% (3.9-6.5) at 7 d[7,15]. After a stroke or TIA, the risk of recurrence is still high in the first month (4%) and year (12%) but falls down to about 5% per year thereafter[15]. For this reason, secondary prevention should be started urgently in the acute phase after TIA or minor stroke. Identifying patients at higher risk of recurrence is, therefore, crucial. For such a purpose several risk scores have been created. Comparison between these scores is hampered by differences in index event, methodology and factors taken into account. To date, the ABCD2 score combined with diffusion-weighted imaging (DWI) data (ABCD2I)[16-19] appears as the best predictor of the early risk of stroke after a TIA. The recently validated ABCD3I score, including carotid stenosis in addition to abnormal DWI, showed even higher predictive power, although c-statistics were less convincing in the validation set[20].
The evidence for acute treatment after TIA and minor stroke is derived from few small randomized trials, extrapolation of results from larger trials in other settings, and on two non-randomized studies of a combination of preventive treatment started urgently[21]. The Early Use of Existing Preventive Strategies for Stroke (EXPRESS) study[22] was nested in a population-based study of all acute TIAs and strokes in the Oxfordshire and compared urgent assessment and treatment (phase 1) with appointment-based clinic assessment and primary care-initiated treatment (phase 2). Results demonstrated a reduction of the 90-d risk of stroke recurrence from 10.3% in phase 1 to 2.1% in phase 2. Similarly, in the SOS-TIA study[23], urgent intensive treatment was associated with a better prognosis and a low rate of recurrent events.
Based on the results of these studies, guidelines now recommend that patients with suspected TIA should be quickly referred to a TIA clinic or to a medical centre providing rapid evaluation and appropriate treatment. In such patients, urgent vascular imaging (ultrasound, cranial tomography angiography or magnetic resonance angiography) in addiction to standardized emergency diagnostic tests, including cranial tomography or magnetic resonance imaging, electrocardiography and laboratory tests are recommended[24,25].
However, the relative contribution of antithrombotic therapy, revascularization techniques, statins and antihypertensive drugs in stroke prevention remains uncertain.
Aspirin is the only antiplatelet drug evaluated in the acute phase of stroke. Given within 48 h of onset of major ischaemic stroke it reduces 14-d morbidity and mortality, mostly by reducing the risk of early recurrent stroke[26]. However, the optimal dose of aspirin has not been studied in head-to-head comparison. A recent meta-analysis by Chen et al[27], including studies in which daily doses of aspirin ranged between 160 to 325 mg, found no heterogeneity of effect on different outcomes. Similarly, optimal timing of initiation of aspirin therapy has not been studied in randomized trials. Though unproven, it seems reasonable to start aspirin therapy as soon as possible, ideally within 48 h of stroke onset. Furthermore, aspirin effect may be negligible in patients at high risk for early recurrence, such as those with stroke or TIA due to arterial thromboembolism[28]. Alternative approaches in these cases, based on the combination of multiple antiplatelet agents with different mechanisms of action, seem, therefore, reasonable. The combination of aspirin and clopidogrel is, actually, more effective than aspirin alone in preventing vascular recurrences and death in acute coronary syndromes[29]. In line with these findings, there is some evidence that a short course of more intensive antiplatelet treatment might be effective also in the acute phase after TIA and minor stroke[21]. Among 731 patients with acute ischaemic stroke or TIA (onset within 3 h) enrolled in five small trials, the addition of clopidogrel to aspirin was associated with a nonsignificant trend toward a reduction in recurrent stroke and an increase in major bleedings[30]. Although dual therapy seems not effective in lacunar strokes[31], it may be more effective than single antiplatelet therapy in preventing early recurrence in patients with acute symptomatic atherothrombosis, according to the recent Clopidogrel in High-risk patients with Acute Nondisabling Cerebrovascular Event trial[32] conducted on a Chinese stroke population. Because of these inconsistencies, the results of three ongoing trials investigating the safety and efficacy of dual and triple antiplatelet therapy[33-35] are required before definite recommendations can be made.
An increase of BP values (even in previously normotensive patients) during the acute phase of ischaemic stroke, followed by a spontaneous decline starting within 90 min of symptoms onset[36] is common finding after stroke. Although extreme arterial hypertension is clearly detrimental, as it might exacerbate oedema and favor the haemorrhagic transformation of ischemia, a moderate increase of BP values during the acute phase of stroke may improve the perfusion of the ischaemic tissue, leading to better outcomes. Conversely, an impairment of cerebral perfusion by excessive BP lowering may exacerbate the ischaemic damage, an effect that is even more evident in conditions of chronically damaged autoregulation, such as in elderly subjects, or in cases of severe carotid stenosis[37]. Unfortunately, the ideal range of BP values to be maintained early after stroke has not yet been determined. Larger trials with well-defined criteria are needed. At present, many clinicians in their daily clinical practice consider a BP greater than 220/120 mmHg an indication to active BP lowering[36]. However, this threshold might be different in cases with minor cerebral damage, such as TIA or minor strokes, where the reduction of cerebral perfusion is likely to be of less concern. In line with this view, antihypertensive therapy started immediately after the acute event did not negatively influence the outcome in the EXPRESS[22] and SOS-TIA[23] studies.
Although antihypertensive medications are effective in the long-term secondary prevention after stroke and TIA[38], the issue of early BP lowering after a cerebrovascular event is still uncertain, recent trials suggesting no benefit[39-41]. In the Controlling Hypertension and Hypotension Immediately Post-Stroke trial[39], oral nimodipine started within 48 h after ischaemic stroke onset in 350 patients was associated with a higher mortality rate and similar functional outcome. The Scandinavian Candesartan Acute Stroke Trial[41], comparing candesartan to placebo given within 30 h of ischaemic or haemorrhagic stroke, showed no difference in the composite endpoint of vascular death, myocardial infarction or stroke after 6-mo follow-up. Similarly, in the Continue or Stop Post-Stroke Antihypertensive Collaborative Study trial[40], continuation of antihypertensive therapy during hospitalization for ischaemic stroke was not associated with a reduction of 6-mo mortality or cardiovascular event rate, when compared to discontinuation of preexisting antihypertensive drugs.
In the acute phase after a cerebrovascular event statins might be beneficial because of their pleiotropic effects. These include a neuroprotective effect, limiting damage and improving recovery (in particular for major strokes), as well as an antithrombotic effect, which in turns may prevent early recurrences (especially in TIAs and non disabling strokes). The benefits related to neuroprotective effects are perhaps more evident in the first 3 to 7 d after stroke and with higher doses of statins, as suggested by some observational studies and clinical data[42-44]. However, data from the fast assessment of stroke and transient ischaemic attack to prevent early recurrence pilot trial are discordant[45]. In this recent trial patients who presented with TIA or minor stroke within 24 h of symptoms onset were randomly assigned to simvastatin or placebo. No evidence of benefit of simvastatin was shown in the primary outcome of recurrent strokes within 90 d. Furthermore, a recent meta-analysis, including 8 randomized controlled trials, found insufficient evidence to establish safety and effectiveness (also in terms of secondary prevention) of statin use in the acute phase after ischaemic stroke[46]. Similarly, initiation of statin therapy within 14 d following the onset of acute coronary syndromes does not reduce significantly the risk of death, myocardial infarction or stroke up to 4 mo[47]. The major ongoing studies aimed at further assessing the benefit of early statin administration mainly focus on functional outcomes instead on the prevention of early recurrences[46].
Hyperglycemia is common during acute ischaemic stroke, being present in more than 40% of patients, mostly among those with a history of diabetes[48,49], and it is associated with worse outcome. So far, only 1 randomized efficacy trial of hyperglycemia treatment in acute stroke, the Glucose-Insulin-Stroke Trial-UK[50], has been reported. Nine hundred and thirty-three patients with acute ischaemic stroke within 24 h of symptom onset, not previously treated with insulin, were randomized to unblended intravenous treatment with insulin, potassium, and glucose versus saline for 24 h. Although the results of this trial in terms of clinical outcomes were neutral, the key questions remain unanswered because of the trial design. Thus, the definitive efficacy and safety of earlier and greater reductions in serum glucose levels during acute ischaemic stroke remain to be studied. In the meantime, it is reasonable to treat hyperglycemia to maintain blood glucose levels in a range of 140 to 180 mg/dL, preventing hypoglycemia with close monitoring, according to the current American Diabetes Association guidelines for glycemic contro[51]. No data are available for the treatment of diabetes in the setting of TIA or minor strokes, but it seems reasonable to apply the same recommendation as in major strokes. Furthermore, information concerning the value of hyperglycemic control in early secondary prevention of stroke, as well as in long-term secondary prevention, is lacking.
Evidence from randomized controlled trials for long-term secondary prevention of ischaemic stroke is more robust than for acute treatment.
Ischaemic stroke is a heterogeneous clinical condition and the end-result of different underlying mechanisms: atherothrombosis (30%), cardioembolism (20%), small-vessel disease (25%). Approximately 25% of cases have no detectable cause (cryptogenic). Elucidating the mechanism of stroke is crucial to select the most appropriate therapeutic strategy and prevent further events.
Cerebral ischaemia of arterial origin: Antiplatelet agents are the cornerstone for secondary prevention of ischaemic events in patients with noncardioembolic strokes. Commonly used antiplatelet agents are aspirin, dipyridamole , and clopidogrel. Aspirin is an irreversible inhibitor of cyclooxygenase-1, which in turn inhibits the formation of thromboxane A2. Dipyridamole increases cyclic AMP by inhibiting platelet phosphodiesterase E5. Clopidogrel is a thienopyridine P2Y12 adenosine diphospate receptor blocker. Ticlopidine, which has the same mechanism of action as clopidogrel, is no longer recommended because of its side effects.
Aspirin, the most commonly used antiplatelet drug, is recommended for secondary prevention after cerebral ischaemia of arterial origin at a dose of 30-325 mg daily[24,25,52]. In order to reduce bleeding complications, after the acute phase (generally 1 or 2 wk after the event) the aspirin dose may be reduced to 75-100 mg[52]. However, in a meta-analysis of trials for secondary prevention of stroke, the relative reduction in stroke risk achieved by aspirin is small, being only 13%[53]. To date, the three large trials[54-56] which investigated whether vitamin K antagonists (VKAs) are more effective than aspirin for this indication, showed no difference, irrespective of the intensity of anticoagulation. Several trials have studied other antiplatelets as an alternative or in addition to aspirin. The second European Stroke Prevention Study (ESPS2) trial[57] and the European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT)[56] investigated the efficacy of the combination of aspirin plus dipyridamole versus aspirin alone. Meta-analysis showed a relative risk reduction of 18% in the vascular events in favour of the combination therapy[58]. In the Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) study, clopidogrel achieved a non-significant 7.3% reduction in relative risk of the composite outcome of stroke, myocardial infarction or vascular death, when compared to aspirin[59]. The combination of aspirin plus clopidogrel was compared with clopidogrel alone in the Aspirin and Clopidogrel compared with Clopidogrel Alone after Recent Ischaemic Stroke or TIA in High-risk Patients (MATCH) trial[60] and with aspirin monotherapy in the Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management, and Avoidance (CHARISMA) trial[61]. Both trials showed higher rates of bleeding complications in the dual antiplatelet arm which overcome any beneficial effect. A more recent study, comparing clopidogrel plus aspirin with aspirin alone in recent lacunar strokes confirmed these results[31]. Although in indirect comparisons the combination of aspirin plus dipyridamole was more effective than clopidogrel[62], the non-inferiority Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial did not confirm this result, showing a similar rate of recurrent stroke in both treatment groups[63]. Furthermore, the rates of discontinuation and of major systemic hemorrhages were higher in the aspirin plus dipyridamole group.
Current therapies have several limitations, including a weak inhibition of platelet function with modest clinical effect, blockade of only one platelet pathway, slow onset of action (i.e., for clopidogrel), high bleeding risk, and interpatient response variability with poor inhibition of platelet activity. The concept of “antiplatelet resistance” has been proposed for those patients experiencing recurrences during correct antiplatelet therapy. It occurs when the drug biochemically fails to inhibit platelet activation, as measured by in vitro platelet function assays. Proposed mechanisms responsible for this phenomenon include drug-drug resistance and genetic polymorphisms of antiplatelet metabolism or drug-receptor site. However, because of the lack of a standardized assay and the poor correlation between antiplatelet resistance and recurrent clinical events, currently there is no indication to screen patients for antiplatelet resistance[64,65]. For clopidogrel, in particular, it seems prudent to avoid interactions of drugs with a well-known effect on hepatic cytochrome p450, as it has been demonstrated that these drugs might affect clopidogrel metabolism[64]. Consequently, in the last years, other antiplatelet agents with different mechanisms of action have been investigated and compared to aspirin (Figure 2). Triflusal is a thromboxane inhibitor structurally related to aspirin with a similar efficacy in preventing vascular events, but a lower risk of bleeding, in spite of more non-haemorrhagic adverse gastrointestinal events[66]. Cilostazol is a phosphodiesterase-3 inhibitor which represents a promising alternative to aspirin in Asian people with stroke. A meta-analysis including two randomized clinical trials concluded that cilostazol is more effective than aspirin in the prevention of vascular events secondary to stroke, causes more frequently minor adverse effects, but less frequently bleeding complications[67]. The Prevention of Cardiovascular Events in Ischaemic Stroke Patients with High Risk of Cerebral Haemorrhage (PICASSO) trial is an ongoing study aimed at randomizing patients with previous stroke and high bleeding risk (i.e., patients with a symptomatic or asymptomatic old cerebral haemorrhage) to aspirin or cilostazol (and to probucol)[68]. Sarpogrelate, a selective inhibitor of 5-hydroxytryptamine receptors, has been investigated in comparison with aspirin and turned out to be non-inferior to the latter for prevention of stroke recurrences, with fewer bleeding events[69]. Terutroban, which is a specific antagonist of the thromboxane A2 receptor, was no more effective than aspirin for prevention of stroke in the large Prevention of Cerebrovascular and Cardiovascular Events of Ischaemic Origin with Terutroban in Patients with a History of Ischaemic Stroke or TIA (PERFORM) trial[70]. Finally, vorapaxar is a novel antiplatelet agent that selectively inhibits the cellular action of the thrombin through antagonism of proteinase activated receptor 1. In a recent trial[71] in which vorapaxar was compared to placebo in patients with vascular events, it reduced the risk of cardiovascular death or ischaemic events in patients with stable atherosclerosis who were receiving standard therapy. However, it increased major bleedings, including intracranial haemorrhages.
On the basis of these trials, international guidelines still vary, but most recommend aspirin plus dipyridamole or clopidogrel as first line therapy in long-term secondary prevention of stroke. If these are not available or contraindicated, aspirin monotherapy, triflusal and cilostazol are alternatives (Table 1). However, even if both aspirin plus dipyridamole and clopidogrel alone are statistically superior compared to aspirin alone, clinical superiority is at best minimal. Furthermore, if tolerance profile is included in judgment of efficiency, then aspirin or clopidogrel monotherapy should be advised as the first-line therapy, since aspirin plus dipyridamole and aspirin plus clopidogrel carry higher rates of discontinuation and of bleeding complications, respectively. If also economic considerations are taken into account, then aspirin therapy remains the best choice[72].
| Options | Recommendation | |
| AHA/ASA[25] | Aspirin (50-325 mg); aspirin plus dipyridamole; clopidogrel | Aspirin, aspirin plus dipyridamole or clopidogrel |
| ESO[24] | Aspirin; aspirin plus dipyridamole; clopidogrel; triflusal | Clopidogrel or aspirin plus dipyridamole |
| ACCP[52] | Aspirin (75-100 mg); clopidogrel; aspirin plus dipyridamole; cilostazol | Clopidogrel or aspirin plus dipyridamole |
Patients who present with a first or recurrent stroke are commonly already on antiplatelet therapy. To date, for patients experiencing a stroke during aspirin therapy, there is no evidence that increasing the dose of aspirin provides additional benefit. Moreover, although alternative antiplatelet agents are often considered, no single agent or combination has been studied. In such a situation it is important to take into account the compliance to therapy, as well as other vascular risk factors and possible different stroke mechanisms.
Cerebral ischaemia caused by atrial fibrillation: Roughly 20% of all TIAs and ischaemic strokes have a cardiac origin, most commonly caused by atrial fibrillation (AF). AF is associated with a five-fold increased risk of stroke, the yearly risk varying from 0.2% in patients with AF alone to more than 10% in individuals with other risk factors[73]. The major risk factors for stroke in AF patients are a history of stroke or TIA, advancing age, hypertension, systolic BP (SBP) > 160 mmHg, and diabetes. These and other risk factors have been used to create several scores for stroke risk calculation, in order to recognize patients who could benefit from anticoagulation. The most widely used model for stratification of stroke risk is CHADS2 score (Table 2). However, although the score is simple and has been externally validated it does not reliably discriminate between patients with low risk, who do not need anticoagulation, and patients at intermediate risk, who do[73]. This has prompted to the development and the external validation in independent cohorts of a new score, the CHA2DS2VASc score (Table 2). Because of the inclusion of newly recognized risk factors for stroke, this score allows a better stroke risk stratification in patients who are considered at low or intermediate risk for stroke[74]. Beside quantification of stroke risk, another important issue is the stratification of bleeding risk associated with anticoagulation. Among the schemes created for such a stratification, the most frequently used is the HAS-BLED score (Table 2), which was originally derived from a cohort of 3456 patients with AF and a one-year follow-up[75]. HAS-BLED score, which has been validated in external cohorts, correlates with the risk of intracranial bleeding, a score of more than 3 identifying patients at high bleeding risk. In these conditions, it does not necessarily mean that anticoagulation should not be initiated but that such therapy warrants special caution, control of modifiable risk factors, and close monitoring[73]. Difficulty arises in clinical practice when patients have key risk factors for both ischaemic stroke and major bleeding (e.g., age, hypertension, or previous stroke). Among these factors, previous stroke and increasing age are more strongly associated with ischaemic stroke than with intracranial bleeding[76,77].
| CHADS2 | |
| Congestive heart failure | 1 |
| Hypertension | 1 |
| Age ≥ 75 yr | 1 |
| Diabetes mellitus | 1 |
| Stroke, TIA, or thromboembolism | 2 |
| Maximum score | 6 |
| CHA2DS2VASc | |
| Congestive heart failure/LV dysfunction | 1 |
| Hypertension | 1 |
| Age ≥ 75 yr | 2 |
| Diabetes mellitus | 1 |
| Stroke, TIA, or thromboembolism | 2 |
| Vascular disease (previous MI, PAD, or aortic plaque) | 1 |
| Age 65-74 yr | 1 |
| Sex category (female sex) | 1 |
| Maximum score | 9 |
| HAS-BLED | |
| Hypertension | 1 |
| Abnormal renal/liver function | 1 point each |
| Stroke | 1 |
| Bleeding history or predisposition | 1 |
| Labile INR | 1 |
| Elderly (age > 65 yr) | 1 |
| Drugs (e.g., concomitant antiplatelet/NSAID) or alcohol | 1 point each |
| Maximum score | 9 |
Recommendations for patients with AF and a history of stroke and TIA are based on the pooled effect of anticoagulation in primary and secondary prevention studies, as data on secondary prevention are limited to few studies. The first of these studies is the European AF Trial[78], in which patients were randomly allocated to VKAs, 300 mg/d-aspirin, or placebo. VKAs turned out to be the most effective treatment, despite a higher number of major haemorrhages. The findings were confirmed in patients with previous TIA or ischaemic stroke enrolled in the Stroke Prevention in AF III trial[79], which was halted early because patients assigned to fixed low-dose VKA (INR 1.2-1.5) plus aspirin 325 mg daily had four times more ischaemic events, compared to those allocated to regular VKA dose. A further trial in patients with recent cerebral ischaemia and AF found 15% reduction of recurrences with VKA than with indobufen[80]. Adjusted-dose warfarin (target INR 2.0-3.0) turned out to be significantly more effective than the combination of aspirin plus clopidogrel in the AF Clopidogrel Trials with Irbesartan for prevention of Vascular Events (ACTIVE)[81,82]. ACTIVE W was stopped prematurely because of an excess of primary outcomes in the aspirin plus clopidogrel group than in warfarin group. ACTIVE A included patients who were not eligible for VKAs and showed a small advantage for the combination of clopidogrel and aspirin compared to aspirin alone in preventing ischaemic events, but with more bleedings.
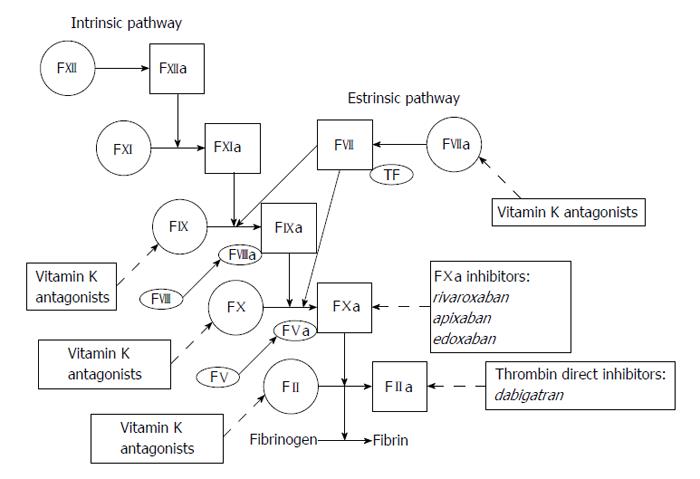
Despite their effectiveness, VKAs have many limitations, including slow onset and offset of action, interindividual variability in their effect, narrow therapeutic index, food and drug interactions and reduced synthesis of all vitamin K-dependent proteins[83]. It has been calculated that at least a third of patients with AF who are at risk for stroke are either not started on VKAs therapy or discontinue the therapy. These limitations have prompted to the development and assessment of other agents that target the coagulation cascade at sites different from those of classic VKAs: factors Xa and direct thrombin inhibitors (Figure 3). The first of these agents, Ximelagatran, a factor Xa inhibitor, was investigated in two trials[84,85]. It resulted as effective as warfarin in prevention of stroke with fewer bleedings, but it turned out to be hepatotoxic and was then withdrew. Dabigatran, a thrombin inhibitor, was compared to dose-adjusted warfarin in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial[86] in doses of 110 and 150 mg twice daily in approximately 12000 patients, 20% of whom had a history of TIA or stroke. The lower dose of dabigatran was non-inferior to warfarin in reducing the rate of stroke or systemic embolism, while the higher dose was superior. Both doses significantly reduced major bleedings compared with warfarin in patients younger than 75 years, whereas in elderly patients the lower dose was associated with a similar rate and the higher dose with an increased rate of major bleedings[87]. The factor Xa inhibitor rivaroxaban (20 mg daily) was compared with warfarin in the Rivaroxaban-Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in AF (ROCKET-AF)[88]. The study population in this trial was at high risk of stroke: 55% of patients had previous stroke or TIA and 90% had either a previous stroke or TIA, or three or more risk factors for stroke (mean CHADS2 score 3.5). Rivaroxaban was non-inferior to warfarin for the prevention of stroke or systemic embolism and had a similar adverse effect profile. In the Apixaban for Reduction in Stroke and Other Thromboembolic Events in AF trial[89] apixaban was better than warfarin in reducing the rate of embolic events and was associated with significantly less major bleedings, less intracranial haemorrhages and lower mortality. Finally, the Apixaban vs Aspirin to Prevent Stroke (AVERROES) trial investigated apixaban (5 mg twice daily) versus aspirin (81-324 mg daily) in patients with AF who were unsuitable or unwilling to receive VKAs[90]. The rate of stroke or systemic embolism was significantly lower with apixaban than with aspirin, whereas the rates of major bleeding, intracranial haemorrhage, gastrointestinal bleeding, and myocardial infarction were similar in both groups. Another large phase III trial with a new anticoagulant, edoxaban, is ongoing[91]. The proportion of patients with AF who were enrolled in all these trials and had a previous stroke or TIA varied between 14% in AVERROES and 55% in ROCKET-AF. In each trial, the absolute rate of stroke or systemic embolism was higher in patients with a previous stroke or TIA than in those without, but the relative effect of each new anticoagulant compared to warfarin in the prevention of stroke or systemic embolism was consistent among patients with or without previous cerebral ischaemic events. Similarly, the relative effect of each new agent on major bleeding did not differ[92-95]. Indirect comparisons suggest that the relative effects of each of the new oral anticoagulants compared with warfarin were consistent for stroke and systemic embolism, haemorrhagic stroke, and mortality, but not for myocardial infarction and extracranial major bleeding[96-102]. Similar results have been confirmed in a recent meta-analysis of data from 12 phase II and phase III randomized controlled trials comparing new oral anticoagulants with warfarin[103].
The majority of current guidelines still regard VKAs at INR 2.0-3.0 to be the standard treatment after cerebral ischaemia of cardiac origin, including AF, for patients who are suitable for such therapy, with few exceptions, on the basis of recent findings concerning new anticoagulants[24,25,52] (Table 3). Although these drugs have some advantages compared to warfarin, the unknown long-term safety profile, the absence of an antidote in the case of bleeding and their costs are important issues to consider.
Long term management of hypertension significantly reduces the risk of recurrent events. A meta-analysis of 7 randomized controlled trials including 15527 patients with TIA or stroke randomly allocated to treatment group within 1-14 mo after the event, showed reduction of recurrent stroke, myocardial infarction and vascular death[38]. Benefit was present irrespective of a previous diagnosis of hypertension and risk reduction was greater with larger BP lowering. However, there is uncertainty regarding the class of drugs to prefer because of the small number of trials included. Significant reductions in recurrent stroke were seen with diuretics alone and in combination with angiotensin-converting enzyme inhibitors (ACEIs) but not with β-blockers (BBs) or ACEIs used alone; nonetheless, statistical power was limited, particularly for the assessment of β-blockers, and calcium channel blockers (CCBs) and angiotensin receptor blockers (ARB) were not evaluated in any of the included trials. One of the largest trial included in the meta-analysis, the PROGRESS trial, showed a 26% reduction of the risk of stroke with the combination of perindopril and indapamide compared to placebo[104]. Patients were included regardless of baseline BP and benefits were seen even in those patients who were normotensive before admission. Since this meta-analysis, 2 additional large-scale randomized trials were published: the Morbidity and Mortality after Stroke, Eprosartan Compared to Nitrendipine for Secondary Prevention (MOSES) trial[105], and the PRoFESS trial[106]. In the MOSES, patients with an ischaemic event in the previous 2 years were randomly allocated to eprosartan (an ARB) or nitrendipine (a CCB). The risk of stroke was lower in the former group, but the benefit was only due to fewer TIAs, with no significant effect on stroke[105]. The PRoFESS failed to show a protective role of telmisartan, an ARB, in stroke prevention. Similarly, another study found no difference in a composite outcome of death from vascular causes, myocardial infarction, stroke or hospitalization for heart failure between patients with previous vascular event of any kind or diabetes randomly allocated to telmisartan or ramipril[107]. The Telmisartan to Prevent Recurrent Stroke and Cardiovascular Events (TRANSCEND) trial[108] showed only a modest effect on prevention of vascular events in patients treated with telmisartan, thus confirming the lack of evidence of ARB effectiveness in stroke prevention.
On the basis of the available evidence, current guidelines recommend antihypertensive treatment in most patients with recent TIA or stroke, beyond the first 24 h, irrespective of a history of hypertension before admission, with some favouring the combination of an ACEI and a diuretic agent, on the basis of PROGRESS trial[25]. An absolute BP target and reduction are uncertain and should be individualized, but benefit has been associated with an average reduction of roughly 10/5 mmHg, and normal BP levels have been defined as > 120/80 mmHg[109].
However, recent studies have shown that visit-to-visit variability in BP and episodic hypertension are powerful risk factors for stroke, independently of mean BP, and that the benefits of some BP-lowering drugs are attributable partly to reduce variability in BP levels[110,111]. Although reductions in mean SBP are similar with different drug classes, CCBs and diuretics also reduce variability in SBP, while BBs increase variability[112,113]. In particular, it has been shown that higher doses of CCBs reduce BP variability more than lower doses, and higher doses of BBs increase BP variability[114]. This means that combinations containing a low dose of BBs will have less adverse effects on variability in SBP and the associated risk of stroke, but low doses of CCBs will be less effective in reducing BP variability. Drug class effects on variability persist even in combination therapy.
Another issue to consider is the heterogeneity of stroke patients. Elderly patients seem to benefit most from a combination therapy of ACEIs and diuretics[115], as well as patients with renal impairment or diabetic nephropathy, in whom ACEIs and ARBs are recommended. The detrimental effect of such drugs on BP variability could be offset by the combination use with CCBs, as suggested in the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension study[116], in whom patients with hypertension and previous vascular disease, including stroke, were randomly allocated to a combination of benazepril plus amlodipine or a combination of benazepril plus hydrochlorothiazide. The former group of patients had fewer combined cardiovascular deaths and chronic kidney disease events compared to the latter.
However, direct comparisons of drugs for secondary prevention of stroke are needed in order to recommend the most appropriate BP-lowering drug regimen. More research is required to identify ideal combinations and doses of drugs for the prevention of stroke in specific patient groups with particular reference to their effect on BP variability.
Although the association between serum lipid concentrations and ischaemic risk is weaker for stroke than for myocardial infarction[117], lipid levels modification is important in both primary and secondary prevention of stroke[118]. A recent observational study[44] showed a better functional outcome in stroke patients who received statin therapy before admission to hospital and who continued such a treatment during hospitalization. The prognosis was even better with higher doses of statins and when therapy was started earlier. Regarding secondary prevention, in a retrospective analysis of the Heart Protection Study (HPS) [119] restricted to subjects with a history of stroke, simvastatin did not significantly reduce the risk of stroke. In contrast, the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial[120] showed a 16% reduction in stroke recurrence in patients with previous stroke or TIA, not of cardiac origin, randomized to atorvastatin 80 mg daily versus placebo, within 6 mo from index event. One concern in the SPARCL trial and in the subset of patients in HPS[119] who had a stroke before randomization was a significant increase in the risk of haemorrhagic stroke in patients allocated to statin treatment[121]. Further analysis showed that much of excess risk of haemorrhagic stroke on atorvastatin therapy was in patients with a small vessel disease stroke as qualifying event, either of haemorrhagic or ischaemic type[122]. Although in the former group there was no overall benefit, in the latter the net benefit was still in favour of treatment because of a reduction in ischaemic recurrences[122]. This suggests that statin therapy should be initiate only in patients with atherosclerotic stroke or in presence of other noncerebrovascular indications, such as coronary heart disease or diabetes[123].
Another important clinical issue relates to low-density lipoprotein (LDL) concentration. In a meta-analysis of all statin trials that looked at intense LDL lowering versus standard treatment, achievement of an LDL concentration of 100 mg/dL resulted in 16% relative reduction in risk of stroke. Subanalysis of the SPARCL trial showed a 28% reduction in stroke risk with a LDL concentration lower than 70 mg/dL in comparison with a level of 100 mg/dL[124,125]. The Treat Stroke to Target trial[126] is an ongoing study aimed at unravelling the issue regarding the optimal LDL target to reach. The working hypothesis of this trial is that the achievement of an LDL concentration of 70 mg/dL is better than 100 mg/dL in patients with previous atherosclerotic TIA or stroke. The Optimal BP and Cholesterol Targets for Preventing Recurrent Stroke in Hypertensive (ESH-CHL-SHOT) trial[127] will be started soon to address this issue. Its purpose, indeed, is to investigate both SBP and LDL cholesterol levels to achieve to optimize secondary prevention. Regarding lipid-lowering treatment in particular, the patients will be randomly allocated to one of two different LDL targets, 70 to 110 mg/dL or < 70 mg/dL, using different statins, including simvastatin, pravastatin, fluvastatin, atorvastatin or rosuvastatin.
Furthermore, there is still uncertainty regarding whether to start with high-dose or low-dose statin therapy, considering benefit and potential side effects. The SPARCL trial[120] reliably showed that high-dose atorvastain at 80 mg/d reduces the risk of stroke and other cardiovascular recurrences in patients with non cardioembolic stroke. It is however unclear, in the absence of randomized comparisons in patients with stroke, if lower doses of atorvastatin are equally or less effective than the high-dose regimen. There is evidence that atorvastatin at 80 mg/d provides significant reductions in the risk of cardiovascular events and stroke beyond that provided by a 10-mg dose in patients with coronary disease[128]. However, the high-dose regimen was complicated more frequently by higher rates of adverse events and discontinuation, both in Treating to New Targets (TNT) trial[128] and SPARCL trial[120]. Moreover, the Food and Drug Administration issued a warning regarding increased risk of myopathy in patients taking simvastatin at 80 mg/d, especially if taken in combination with CCBs, which are frequently used in patients with stroke. Although the SPARCL results support the use of high-dose atorvastatin for secondary prevention of stroke, it is unclear whether the highest currently approved doses of statins have neuroprotective effects. It is also unknown if other hydroxymethylglutaryl-coenzymeA reductase inhibitors, in different doses, could be potentially effective in prevention of stroke recurrences. In this regard, two trials are ongoing to investigate the combined effect of this class of drugs[127] and that of pravastatin[129] in particular in long-term secondary prevention after stroke.
On the basis of available data the American Heart Association/American Stroke Association (AHA/ASA) guidelines recommend statin therapy with intensive lipid-lowering effect for secondary prevention among patients with ischemic stroke or TIA who have evidence of atherosclerosis, an LDL-level ≥ 100 mg/dL, and who are without known coronary heart disease. They suggest, as a target, a reduction of at least 50% in LDL or, alternatively, an LDL level of < 70 mg/dL[25].
Other medications used to treat dyslipidemia include niacin, fibrates, and cholesterol absorption inhibitors. These agents can be used by patients intolerant to statins, but the evidence of their efficacy for prevention of stroke recurrence is scarce. Niacin has been associated with a reduction in cerebrovascular events[130], whereas gemfibrozil reduced the rate of total strokes among men with coronary artery disease and low levels of high-density lipoprotein cholesterol (≤ 40 mg/dL) in the Veterans Affairs HDL Intervention Trial. The latter result was no more significant when adjudicated events alone were analyzed[131].
Percutaneous PFO closure: PFO is a common embryonic defect of the interatrial septum, being present in up to 25% of healthy people. In a meta-analysis PFO was found to be significantly associated with increased risk of stroke in younger patients with cryptogenic stroke, especially when atrial septal aneurysm (ASA) is also present[132]. This leads to the assumption that PFO may be the cause somehow associated to stroke occurrence, although it may also be an incidental finding[133]. The role of PFO in determining the risk of recurrence is uncertain, as well. In a substudy of the PFO in Cryptogenic Stroke Study (PICSS)[134] there were no differences in the rates of recurrent strokes in those with or without PFO, as well as no demonstrated effect on outcomes based on PFO size or presence of ASA. In contrast, the study of Mas et al[135] reported higher recurrence rates of stroke associated with both PFO and ASA, indicating that the presence of both these cardiac abnormalities was a predictor of an increased risk of recurrent stroke, whereas isolated PFO, whether small or large, was not. Thus, the natural history after cerebrovascular events in patients with PFO remains insufficiently defined. As a consequence, the optimal management strategy for treating patients with cryptogenic stroke who are discovered to have a PFO remains to be determined. Observational studies on medical treatment in patients with PFO with either antiplatelets or warfarin reported a risk of recurrent stroke or TIA ranging from 3% to 12% during the first year[136]. Both larger PFO size and a greater degree of right-to-left shunt increased the risk of paradoxical embolism in one study[136]. Of note, observational data for the comparative effectiveness of anticoagulants versus antiplatelet agents for secondary prevention after stroke associated to PFO show a significant benefit of warfarin, whereas the only relevant randomized study, coming from a subgroup of PICSS[134], found a non-significant trend in favor of warfarin over aspirin. On the basis of this evidence, AHA guidelines state that there are insufficient data to establish the superiority of warfarin over aspirin for secondary stroke prevention after stroke or TIA in patients with PFO[25]. However, warfarin might be more beneficial in presence of deep venous thrombosis or ASA, according to the European Stroke Organization guidelines[24]. Another therapeutic possibility is percutaneous PFO closure, which has supplanted surgical procedures. Case series and nonrandomized comparisons have long suggested that closure is a highly efficacious procedure and have led to the rapid off-label adoption of this intervention[137]. In contrast, recent randomized controlled trials failed to identify any statistically significant difference between closure and medical treatment for secondary prevention after stroke[138-140]. However, such trials have several limitations and low statistical power. In the Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or TIA due to Presumed Paradoxical Embolism through a PFO (CLOSURE) trial[138] the main limitation was the low outcome rate, compared to previous observational studies, thus pointing to a failure to select patients with PFO-related events. This may, in part, be attributable to a reluctance to randomize those patients with cryptogenic stroke who appear most likely to have had a PFO-related event (i.e., patients with clinical indicators of paradoxical embolism, such as large shunt, associated ASA, Valsalva maneuver at onset of stroke, or absence of any conventional stroke risk factors). Other limitations of this trial include idiosyncratic effects, such as in situ thrombosis or other mechanical complications, with the use of the obsolete STARFlex device, which may have increased the outcome rates in the intervention arm, as well as the short follow-up, that may be inadequate to capture the benefit of the intervention[137]. The Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT) trial[139] confirmed the results of the CLOSURE trial in intention-to-treat analysis, in spite of important differences between the two studies with respect to the study design, the population included, and the device tested. In the RESPECT trial[139] the enrollment criteria were more stringent and the follow-up period was longer than in the CLOSURE. Moreover, the Amplatzer PFO Occluder, as compared to STARFlex used in the CLOSURE, was associated with higher effective closure rate. Finally, although different in terms of population and endpoints, the Percutaneous Closure of PFO in Cryptogenic Stroke (PC) trial[140] corroborated previous results. However, unlike the results of the CLOSURE trial, the results of the RESPECT and the PC trials have encouraged the supporters of PFO closure. The advocates of closure focus on the modest statistical power of these trials, the substantial relative effect size of the point estimates in both trials, the significance of the per-protocol and as-treated analyses in the RESPECT, and arbitrariness of the conventional P-value threshold of 0.05. Although the controversy over efficacy may not be settled, it is worth noting that the safety profile of Amplatzer device appeared to be superior to that of STARFLex device tested in the CLOSURE. This supports the hypothesis that PFO closure, in certain conditions, could be superior to medical treatment and reinforces the need to carry on trials with more selected populations. Indeed, other trials are ongoing, such as the PFO Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) trial[141] and the GORE® HELEX® Septal Occluder/GORE® Septal Occluder for PFO Closure in Stroke Patients (REDUCE) trial[142]. However, like previous analyses, these new studies are hampered by a slow and skewed enrollment since most patients assumed to be at high risk undergo percutaneous closure. Beside, stroke pathophysiology is multifactorial, and the diagnosis of PFO-mediated paradoxical embolism is presumptive. Both a PFO and a cryptogenic stroke may coexist without causal relation in a given patient. In this case, obviously, PFO closure will not reduce the risk of recurrence. Current guidelines, which are antecedent to these recent trials, do not recommend closure because of the paucity of data[25]. However, pending other trials and based on RESPECT, it would be reasonable to discuss closure with Amplatzer device in young patients (< 50 years) with a substantial shunt and a cortical infarct, in the absence of known vascular risk factors[143].
Beside medical therapy, the mainstays of treatment options for symptomatic large-artery atherosclerosis are carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAS), the latter being emerged more recently as an alternative to surgical intervention to prevent recurrent strokes.
CEA plus medical therapy is more effective and safe than medical therapy alone in secondary prevention after stroke for symptomatic patients with > 70% carotid stenosis according to 3 major trials, the European Carotid Surgery Trial[144], the North American Symptomatic CEA Trial[145], and the Veterans Affairs Cooperative Study[146]. However, in all these trials medical therapy did not include aggressive atherosclerotic medical management based on statins, antihypertensive agents, antiplatelets other than aspirin, and smoking cessation, thus meaning that the benefit of surgery today may be less than in the early trials. Uncertainty exists for patients with symptomatic stenosis in the range of 50% to 69%, for whom CEA should be considered only with appropriate case selection when the risk-benefit ratio is favorable for the patient. The timing of CEA after an acute event is controversial, with experts advocating waiting anywhere from 2 to 6 wk[25]. However, early surgery (< 2 wk) turned out to be beneficial, according to pooled analysis of trials. Furthermore, early intervention (< 3 wk) may be beneficial and safe in low-risk patients with TIA and minor strokes, and in those without haemorrhagic transformation[25].
CAS is a percutaneous, less invasive procedure that has emerged as an interesting alternative to CEA for the treatment of extracranial carotid artery disease, especially thanks to the advances in endovascular technology, including embolic protection devices (EPD) and improved stent design. Complication rates are comparable to CEA. Although advantages related to the less invasive procedure, CAS durability remains unproven. The largest randomized trial in symptomatic patients, the Carotid and Vertebral Artery Transluminal Angioplasty Study 2 trial[147], showed comparable outcome between the two procedures, also in the long-term follow-up. The Stent-Supported Percutaneous Angioplasty of the Carotid Artery Versus Endarterectomy trial[148] which was ended after the second interim analysis owing to the low rate of recruitment, showed similar rates of periprocedural stroke or death and ipsilateral ischaemic stroke up to 2 years between CEA and CAS. However, the fact that only 27% of patients with CAS in this study used EPD may have skewed the outcome. Because EPD reduce periprocedural stroke rates, they have been required in all stenting trials since then. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy trial[149] randomized both symptomatic and asymptomatic high risk patients, showing that CAS was not inferior to CEA. However, a high percentage of patients were asymptomatic (70%), thus limiting the applicability of the results to symptomatic ones. The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis trial[150] showed higher rates of periprocedural stroke or death at 30 d and within 4 years, as well. However, the results of this trial are influenced by the limited experience of the operators, the use of five different stents and seven different cerebral protection devices, and the fact that EPD were optional in the early phase of the study. The most important trial on this topic is the Carotid Revascularization Endarterectomy Versus Stenting Trial[151], which included patients with both symptomatic and asymptomatic carotid disease in similar proportions. It demonstrated that both procedures had similar safety and efficacy profiles for all patients, according to gender and the presence or not of previous cerebrovascular events. However, patients aged 70 years or older had better outcomes with CEA for the primary endpoint (any stroke, death or myocardial infarction (MI) within 30 d and ipsilateral stroke during long-term follow-up) and less adverse events. Younger patients had greater benefit from CAS than older subjects. The CAS group had a greater percentage of patients with stroke within the first 30 d after the procedure, but a lower rate of MI events. In trials of symptomatic patients, stenting was associated with a significantly higher risk of 30-d stroke or death, particularly in people over 70 years, compared to CEA, whereas the long-term rate of ipsilateral stroke were similar for both interventions[152]. However, stenting was associated with less MI and cranial nerve palsies.
On the basis of these studies, current AHA/ASA guidelines recommend that patients who have experienced a TIA or stroke within the past 6 mo and have ipsilateral carotid stenosis of 70%-90% would be candidates for CEA if the perioperative morbidity and mortality risk is less than 6%. In symptomatic patients with a 50%-60% stenosis, the decision to perform CEA depends on factors such as age, comorbidities, and sex. If a patient is a good candidate for CEA, intervention within 2 wk is recommended[25]. To date, CAS is considered an alternative option for symptomatic patients with carotid stenosis of more than 70% as determined by noninvasive imaging or more than 50% as determined by catheter angiography. CAS is also an alternative in patients deemed at high risk for surgical intervention. High risk is defined by presence of severe comorbidites and challenging anatomical features (such as prior neck intervention or irradiation, postendarterectomy restenosis, surgical inaccessible lesion, contralateral carotid occlusion, contralateral vocal cord palsy, or the presence of tracheostomy)[25]. Patients are also recommended to optimize medical therapy with antiplatelets, statins and risk factor modifications.
According to the Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS) trial[153], angioplasty and stenting of intracranial stenosis did not reduce the risk of recurrent stroke compared with aggressive medical therapy, including dual antiplatelets, statins and antihypertensive agents.
Secondary prevention with antiplatelet and antihypertensives medications, statins and anticoagulants as appropriate, should be initiated urgently after TIA or minor strokes because of the high risk of early stroke recurrence. For long-term prevention, aspirin plus dipyridamole or clopidogrel must be proposed as first-line approach after cerebral ischaemia of arterial origin. For cardioembolic strokes in patients with non-valvular AF new anticoagulation agents are challenging the current standards of VKAs. Lipid and blood-pressure-lowering treatments are warranted in all cerebral ischaemia, but recommendations about optimal drug regimen, including doses and when to start therapy, as well as target levels of LDL or BP to be achieved, are still debated. Revascularization procedures, including CEA or stenting, must be taken into consideration early after the event in patients with stroke or TIA related to carotid atherosclerotic disease.
P- Reviewers: Altamura C, Takeuchi N, Wang J S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Murray CJ, Lopez AD, Black R, Mathers CD, Shibuya K, Ezzati M, Salomon JA, Michaud CM, Walker N, Vos T. Global burden of disease 2005: call for collaborators. Lancet. 2007;370:109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366:1773-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 665] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 3. | Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1717] [Cited by in RCA: 1900] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 4. | O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 2163] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 5. | Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, Gutnikov SA, Edwards P, Mant D, Sackley CM. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet. 2004;363:1925-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 730] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 6. | Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 478] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 7. | Hong KS, Yegiaian S, Lee M, Lee J, Saver JL. Declining stroke and vascular event recurrence rates in secondary prevention trials over the past 50 years and consequences for current trial design. Circulation. 2011;123:2111-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, Stewart-Wynne EG. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998;29:2491-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke. 1994;25:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 346] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 10. | Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 273] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Sacco RL, Shi T, Zamanillo MC, Kargman DE. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology. 1994;44:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 307] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke. 2003;34:1457-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901-2906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 859] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 14. | Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Hankey GJ. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis. 2003;16 Suppl 1:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Lemmens R, Smet S, Thijs VN. Clinical scores for predicting recurrence after transient ischemic attack or stroke: how good are they. Stroke. 2013;44:1198-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Cucchiara BL, Messe SR, Sansing L, MacKenzie L, Taylor RA, Pacelli J, Shah Q, Pollak ES, Kasner SE. D-dimer, magnetic resonance imaging diffusion-weighted imaging, and ABCD2 score for transient ischemic attack risk stratification. J Stroke Cerebrovasc Dis. 2009;18:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Ay H, Arsava EM, Johnston SC, Vangel M, Schwamm LH, Furie KL, Koroshetz WJ, Sorensen AG. Clinical- and imaging-based prediction of stroke risk after transient ischemic attack: the CIP model. Stroke. 2009;40:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Calvet D, Touzé E, Oppenheim C, Turc G, Meder JF, Mas JL. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke. 2009;40:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Coutts SB, Eliasziw M, Hill MD, Scott JN, Subramaniam S, Buchan AM, Demchuk AM. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int J Stroke. 2008;3:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet. 2011;377:1681-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, Lovelock CE, Binney LE, Bull LM, Cuthbertson FC. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 799] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 23. | Lavallée PC, Meseguer E, Abboud H, Cabrejo L, Olivot JM, Simon O, Mazighi M, Nifle C, Niclot P, Lapergue B. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 455] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 24. | European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1696] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 25. | Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1154] [Cited by in RCA: 1145] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 26. | Sandercock P, Gubitz G, Foley P, Counsell C. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2003;CD000029. [PubMed] |
| 27. | Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, Xie JX, Warlow C, Peto R. Indications for early aspirin use in acute ischemic stroke : A combined analysis of 40 000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31:1240-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 399] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 531] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 29. | Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4702] [Cited by in RCA: 4480] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 30. | Geeganage CM, Diener HC, Algra A, Chen C, Topol EJ, Dengler R, Markus HS, Bath MW, Bath PM. Dual or mono antiplatelet therapy for patients with acute ischemic stroke or transient ischemic attack: systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 501] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1209] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 33. | Wang Y; ClinicalTrials. gov. Platelet-oriented inhibition in new tia and minor ischemic stroke (POINT) trial. Available from: http: //clinicaltrials.Gov/ct2/show/nct00991029Term=nct00991029&rank=1 (accessed may 31, 2013). |
| 34. | Wang Y; ClinicalTrials. gov. Combination of clopidogrel and aspirin for prevention of early recurrence in acute attherothrombotic stroke (COMPRESS). Available from: http: //clinicaltrials.Gov/ct2/show/nct00814268Term=n-ct00814268&rank=1 (accessed may 31, 2013).. |
| 35. | Wang Y; ClinicalTrials. gov. Triple antiplatelets for reducing dependency after ischaemic stroke (TARDIS). Available from: http: //clinicaltrials.Gov/ct2/show/nct01661322Term=tardis&rank=1 (accessed may 31, 2013). |
| 36. | Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Qureshi AI, Rosenfield K. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3356] [Cited by in RCA: 3285] [Article Influence: 273.8] [Reference Citation Analysis (0)] |
| 37. | Castilla-Guerra L, Fernández-Moreno Mdel C. Update on the management of hypertension for secondary stroke prevention. Eur Neurol. 2012;68:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 332] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 39. | Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, Jagger C. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol. 2009;8:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 40. | Robinson TG, Potter JF, Ford GA, Bulpitt CJ, Chernova J, Jagger C, James MA, Knight J, Markus HS, Mistri AK. Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol. 2010;9:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Sandset EC, Bath PM, Boysen G, Jatuzis D, Kõrv J, Lüders S, Murray GD, Richter PS, Roine RO, Terént A. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 42. | Alvarez-Sabín J, Huertas R, Quintana M, Rubiera M, Delgado P, Ribó M, Molina CA, Montaner J. Prior statin use may be associated with improved stroke outcome after tissue plasminogen activator. Stroke. 2007;38:1076-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L. Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist. 2007;13:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Flint AC, Kamel H, Navi BB, Rao VA, Faigeles BS, Conell C, Klingman JG, Sidney S, Hills NK, Sorel M. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke. 2012;43:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 385] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 46. | Squizzato A, Romualdi E, Dentali F, Ageno W. Statins for acute ischemic stroke. Cochrane Database Syst Rev. 2011;CD007551. [PubMed] |
| 47. | Briel M, Schwartz GG, Thompson PL, de Lemos JA, Blazing MA, van Es GA, Kayikçioglu M, Arntz HR, den Hartog FR, Veeger NJ. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA. 2006;295:2046-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Gentile NT, Seftchick MW, Huynh T, Kruus LK, Gaughan J. Decreased mortality by normalizing blood glucose after acute ischemic stroke. Acad Emerg Med. 2006;13:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 50. | Ribo M, Molina CA, Delgado P, Rubiera M, Delgado-Mederos R, Rovira A, Munuera J, Alvarez-Sabin J. Hyperglycemia during ischemia rapidly accelerates brain damage in stroke patients treated with tPA. J Cereb Blood Flow Metab. 2007;27:1616-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33 Suppl 1:S11-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2231] [Cited by in RCA: 2278] [Article Influence: 151.9] [Reference Citation Analysis (1)] |
| 52. | Lansberg MG, O’Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e601S-e636S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 53. | Algra A, van Gijn J. Cumulative meta-analysis of aspirin efficacy after cerebral ischaemia of arterial origin. J Neurol Neurosurg Psychiatry. 1999;66:255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin. The Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group. Ann Neurol. 1997;42:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 298] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 55. | Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 804] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 56. | Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 605] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 57. | Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1081] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 58. | Halkes PH, Gray LJ, Bath PM, Diener HC, Guiraud-Chaumeil B, Yatsu FM, Algra A. Dipyridamole plus aspirin versus aspirin alone in secondary prevention after TIA or stroke: a meta-analysis by risk. J Neurol Neurosurg Psychiatry. 2008;79:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4584] [Cited by in RCA: 4159] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 60. | Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1618] [Cited by in RCA: 1491] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 61. | Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2037] [Cited by in RCA: 1855] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 62. | Thijs V, Lemmens R, Fieuws S. Network meta-analysis: simultaneous meta-analysis of common antiplatelet regimens after transient ischaemic attack or stroke. Eur Heart J. 2008;29:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 666] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 64. | Field TS, Benavente OR. Current status of antiplatelet agents to prevent stroke. Curr Neurol Neurosci Rep. 2011;11:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Yip S, Benavente O. Antiplatelet agents for stroke prevention. Neurotherapeutics. 2011;8:475-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Costa J, Ferro JM, Matias-Guiu J, Alvarez-Sabin J, Torres F. Triflusal for preventing serious vascular events in people at high risk. Cochrane Database Syst Rev. 2005;CD004296. [PubMed] |
| 67. | Kamal AK, Naqvi I, Husain MR, Khealani BA. Cilostazol versus aspirin for secondary prevention of vascular events after stroke of arterial origin. Cochrane Database Syst Rev. 2011;CD008076. [PubMed] |
| 68. | Kamal AK; ClinicalTrials. gov. Prevention of cardiovascular events in ischemic stroke patients with high risk of cerebral hemorrhage (PICASSO). Available from: http: //clinicaltrials.Gov/ct2/show/study/nct01013532Term=picasso&rank=1 (accessed may 31, 2013). |
| 69. | Shinohara Y, Nishimaru K, Sawada T, Terashi A, Handa S, Hirai S, Hayashi K, Tohgi H, Fukuuchi Y, Uchiyama S. Sarpogrelate-Aspirin Comparative Clinical Study for Efficacy and Safety in Secondary Prevention of Cerebral Infarction (S-ACCESS): A randomized, double-blind, aspirin-controlled trial. Stroke. 2008;39:1827-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Bousser MG, Amarenco P, Chamorro A, Fisher M, Ford I, Fox KM, Hennerici MG, Mattle HP, Rothwell PM, de Cordoüe A. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet. 2011;377:2013-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 71. | Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 721] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 72. | Vanstreels L, Molenberghs G, Voigt JU. Secondary stroke prevention: misguided by guidelines. Acta Cardiol. 2012;67:431-438. [PubMed] |
| 73. | Alberts MJ, Eikelboom JW, Hankey GJ. Antithrombotic therapy for stroke prevention in non-valvular atrial fibrillation. Lancet Neurol. 2012;11:1066-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 967] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 75. | Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2971] [Cited by in RCA: 3328] [Article Influence: 221.9] [Reference Citation Analysis (0)] |
| 76. | Oldgren J, Alings M, Darius H, Diener HC, Eikelboom J, Ezekowitz MD, Kamensky G, Reilly PA, Yang S, Yusuf S. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med. 2011;155:660-67, W204. [PubMed] |
| 77. | McGrath ER, Kapral MK, Fang J, Eikelboom JW, ó Conghaile A, Canavan M, O’Donnell MJ. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2012;43:2048-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262. [PubMed] |
| 79. | Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 700] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 80. | Morocutti C, Amabile G, Fattapposta F, Nicolosi A, Matteoli S, Trappolini M, Cataldo G, Milanesi G, Lavezzari M, Pamparana F. Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (Studio Italiano Fibrillazione Atriale) Investigators. Stroke. 1997;28:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1352] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 82. | Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, Yusuf S. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 781] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 83. | Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:160S-198S. [PubMed] |
| 84. | Albers GW, Diener HC, Frison L, Grind M, Nevinson M, Partridge S, Halperin JL, Horrow J, Olsson SB, Petersen P. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005;293:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 478] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 85. | Olsson SB. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 510] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 86. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7917] [Cited by in RCA: 8030] [Article Influence: 501.9] [Reference Citation Analysis (0)] |
| 87. | Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 807] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 88. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6519] [Cited by in RCA: 6871] [Article Influence: 490.8] [Reference Citation Analysis (2)] |
| 89. | Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6075] [Cited by in RCA: 6503] [Article Influence: 464.5] [Reference Citation Analysis (0)] |
| 90. | Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1747] [Cited by in RCA: 1772] [Article Influence: 126.6] [Reference Citation Analysis (0)] |
| 91. | Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, Hanyok J, Patel I, Shi M, Salazar D. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J. 2010;160:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 363] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 92. | Diener HC, Connolly SJ, Ezekowitz MD, Wallentin L, Reilly PA, Yang S, Xavier D, Di Pasquale G, Yusuf S. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 93. | Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, Diener HC, Donnan GA, Halperin JL, Mahaffey KW. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 94. | Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, Alings M, Goto S, Lewis BS, Rosenqvist M. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 95. | Diener HC, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, O’Donnell M, Hohnloser SH, Hankey GJ, Shestakovska O. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol. 2012;11:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 96. | Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 309] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 97. | Lip GY, Larsen TB, Skjøth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 98. | Schneeweiss S, Gagne JJ, Patrick AR, Choudhry NK, Avorn J. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 99. | Harenberg J, Marx S, Diener HC, Lip GY, Marder VJ, Wehling M, Weiss C. Comparison of efficacy and safety of dabigatran, rivaroxaban and apixaban in patients with atrial fibrillation using network meta-analysis. Int Angiol. 2012;31:330-339. [PubMed] |
| 100. | Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost. 2012;108:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 101. | Testa L, Agnifili M, Latini RA, Mattioli R, Lanotte S, De Marco F, Oreglia J, Latib A, Pizzocri S, Laudisa ML. Adjusted indirect comparison of new oral anticoagulants for stroke prevention in atrial fibrillation. QJM. 2012;105:949-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 103. | Dentali F, Riva N, Crowther M, Turpie AG, Lip GY, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 104. | PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2266] [Cited by in RCA: 2044] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 105. | Schrader J, Lüders S, Kulschewski A, Hammersen F, Plate K, Berger J, Zidek W, Dominiak P, Diener HC. Morbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES). Stroke. 2005;36:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 501] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 106. | Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 603] [Cited by in RCA: 525] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 107. | Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2381] [Article Influence: 140.1] [Reference Citation Analysis (0)] |
| 108. | Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 109. | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13416] [Cited by in RCA: 13258] [Article Influence: 602.6] [Reference Citation Analysis (0)] |
| 110. | Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 539] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 111. | Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1294] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 112. | Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 564] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 113. | Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 519] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 114. | Webb AJ, Rothwell PM. Effect of dose and combination of antihypertensives on interindividual blood pressure variability: a systematic review. Stroke. 2011;42:2860-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 115. | Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2395] [Cited by in RCA: 2084] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 116. | Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 117. | Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1742] [Cited by in RCA: 1561] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 118. | Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5048] [Cited by in RCA: 4630] [Article Influence: 308.7] [Reference Citation Analysis (1)] |
| 119. | Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 721] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 120. | Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 1869] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 121. | Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 469] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 122. | Goldstein LB, Amarenco P, Szarek M, Callahan A, Hennerici M, Sillesen H, Zivin JA, Welch KM. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70:2364-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 305] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 123. | Furie KL. High-dose statins should only be used in atherosclerotic strokes. Stroke. 2012;43:1994-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Amarenco P, Goldstein LB, Callahan A, Sillesen H, Hennerici MG, O’Neill BJ, Rudolph AE, Simunovic L, Zivin JA, Welch KM. Baseline blood pressure, low- and high-density lipoproteins, and triglycerides and the risk of vascular events in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Atherosclerosis. 2009;204:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 125. | Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, Hennerici M, Simunovic L, Zivin JA, Welch KM. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2007;38:3198-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 126. | Amarenco P; ClinicalTrials. gov. Treat stroke to target (TST). Available from: http: //clinicaltrials.Gov/ct2/show/nct01252875Term=nct01252875&rank=1 (accessed may 31, 2013). |
| 127. | Amarenco P; ClinicalTrials. gov. Optimal blood pressure and cholesterol targets for preventing recurrent stroke in hypertensive (ESH-CHL-SHOT). Available from: http: //clinicaltrials.Gov/ct2/show/nct01563731Term=esh-chl-shot&rank=1 (accessed may 31, 2013). |
| 128. | LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2483] [Cited by in RCA: 2451] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 129. | LaRosa JC; ClinicalTrials. gov. Japan statin treatment against recurrent stroke (J-STARS). Available from: http: //clinicaltrials.Gov/ct2/show/nct00221104Term=nct00221104&rank=1 (accessed may 31, 2013). |
| 130. | Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1109] [Cited by in RCA: 910] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 131. | Bloomfield Rubins H, Davenport J, Babikian V, Brass LM, Collins D, Wexler L, Wagner S, Papademetriou V, Rutan G, Robins SJ. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT). Circulation. 2001;103:2828-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 132. | Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 641] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 133. | Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic. Stroke. 2009;40:2349-2355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 269] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 134. | Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 646] [Article Influence: 28.1] [Reference Citation Analysis (1)] |
| 135. | Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 875] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 136. | Meier B, Frank B, Wahl A, Diener HC. Secondary stroke prevention: patent foramen ovale, aortic plaque, and carotid stenosis. Eur Heart J. 2012;33:705-13, 713a, 713b. [PubMed] |
| 137. | Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 138. | Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 707] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 139. | Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 663] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 140. | Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 628] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 141. | Meier B; ClinicalTrials. gov. Patent foramen ovale closure or anticoagulants versus antiplatelet therapy to prevent stroke recurrence (CLOSE). Available from: http: //clinicaltrials.Gov/ct2/show/nct00562289Term=nct00562289&rank1 (accessed july 31, 2013). |
| 142. | Meier B; ClinicalTrials. gov. Gore® helex® septal occluder / gore® septal occluder for patent foramen ovale (pfo) closure in stroke patients - the gore reduce clinical study (hlx 06-03). Available from: http: //clinicaltrials.Gov/ct2/show/nct00730094Term=nct00730094&rank1 (accessed july 31, 2013). |
| 143. | Furlan AJ, Jauss M. Patent foramen ovale and cryptogenic stroke: the hole story. Stroke. 2013;44:2676-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 144. | MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet. 1991;337:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 2164] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 145. | North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6331] [Cited by in RCA: 5711] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 146. | Mayberg MR, Wilson SE, Yatsu F, Weiss DG, Messina L, Hershey LA, Colling C, Eskridge J, Deykin D, Winn HR. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA. 1991;266:3289-3294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 499] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 147. | Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 854] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 148. | Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 943] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 149. | Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2219] [Cited by in RCA: 1888] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 150. | Cambria RP. Commentary on: Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006; 355: 1660-1671. Perspect Vasc Surg Endovasc Ther. 2007;19:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 151. | Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2408] [Cited by in RCA: 2069] [Article Influence: 137.9] [Reference Citation Analysis (0)] |
| 152. | Bonati LH, Lyrer P, Ederle J, Featherstone R, Brown MM. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2012;9:CD000515. [PubMed] |
| 153. | Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1332] [Article Influence: 95.1] [Reference Citation Analysis (0)] |